It offers a secure and practical packaging solution that guarantees the tablets' integrity as well as their safety and effectiveness throughout the entirety of their shelf life. This solution is provided to ensure that the tablets can be stored safely.
This answer is offered as a means of ensuring that the tablets can be stored in a secure manner.
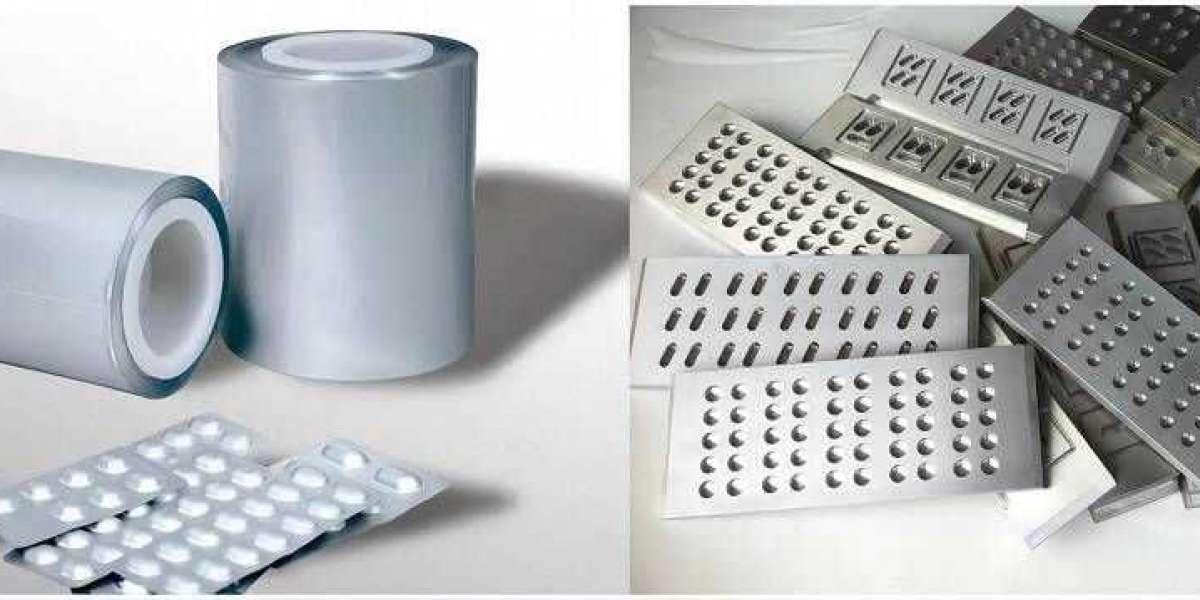
The tablets are protected from environmental factors such as air, light, and moisture by the blister pack, which prevents the tablets from deteriorating or becoming less effective as a result of these factors. These components might make the tablets less effective in their intended purpose.
After this stage of the process is finished, the blister pack will be hermetically sealed using either aluminum foil or a lidding substance, depending on which option was chosen. After this step, the blister packs can be packaged for delivery in cartons that have labels affixed to them. These cartons can then be shipped to the customer. After that, the cartons can be packaged up and sent to the customer. Blister packaging offers a number of benefits to manufacturers who are in the business of producing tablets, and these benefits can be utilized by manufacturers. When patients utilize this service, aluminium blister packaging is much simpler for them to identify themselves and keep track of the dosages that they are supposed to be taking because each patient receives their own individual unit-dose packaging. Patients may find that all that is required to improve their ability to adhere to the treatment regimens that have been prescribed for them is to simply keep a log of the medications that they take.
Blister packaging offers a number of benefits to manufacturers who are in the business of producing tablets, and these benefits can be utilized by manufacturers. When patients utilize this service, aluminium blister packaging is much simpler for them to identify themselves and keep track of the dosages that they are supposed to be taking because each patient receives their own individual unit-dose packaging. Patients may find that all that is required to improve their ability to adhere to the treatment regimens that have been prescribed for them is to simply keep a log of the medications that they take.
In addition, the ability of blister packaging to facilitate automated packing procedures and reduce the likelihood of errors caused by human intervention contributes to an increase in the overall productivity of pharmaceutical manufacturers, which in turn contributes to an improvement in the availability of pharmaceuticals at lower costs. Because of this capability, pharmaceutical manufacturers have been able to significantly increase their overall output. Those that are included in the list that is going to follow are the ones that are the most common.
Blister Packs that are Produced Through the Use of a Cold Form
Tablets that are easily harmed by contact with moisture are typically packaged using the cold-form blister method. This method of packaging is made possible by the use of cold-form blisters.
After that, a lidding material, such as aluminum foil or a laminate, is placed on top of the blister, and the package is then sealed. At long last, the blister is prepared for its intended purpose. It is built with a number of additional features, such as complex opening procedures or specialized locking mechanisms, in order to provide the user with the highest level of protection that is reasonably achievable.
Blister packs with labels that can be easily removed by peeling them off
Users can quickly and easily access the tablet that is contained within peel-off blister packaging by simply peeling back the foil or lidding material. This makes it possible for users to access the tablet in a timely manner. This enables the tablet to be accessed in a speedy and convenient manner.
Calendars, each one contained within its own individual blister pack
A calendar blister packaging is a type of packaging that contains multiple cavities that are organized in the form of a calendar. Each cavity in the calendar blister packaging represents a specific day or dosage of the medication that is contained within it. This specific kind of packaging is referred to as calendar blister packaging, which is another name for it. Almost always, medications that must be taken in accordance with a predetermined dosing schedule are packaged in containers of this kind.
These are some of the most common variations of the blister packaging that are used for a variety of different tablet products.
Components of the blister packaging that are specific to the tablet
The barrier properties offered by PVC, which are effective against oxygen, light, and moisture, protect the tablets from deterioration. This keeps the tablets in good condition for as long as possible. Because of this, the tablets are more likely to maintain their pristine condition. PVDC, which is also known as polyvinylidene chloride, is a material that is utilized quite frequently in the process of coating blister packaging. This process is known as the "blister coating" process. It is widely acknowledged that the best method for sealing blister packs is to make use of films that have been coated with PVDC. This is the material that is used for the blister pack lid.
PET (Polyethylene Terephthalate): PET, which is also known as polyethylene terephthalate, is a material that is lightweight in addition to having the ability to see through it. In blister packaging, it is utilized for the components that make up the blister as well as the covering that goes on top of the blister. Packaging in the form of a blister card is an example of this type of packaging. cyclic olefin copolymer is what the letters COC stand for when they are abbreviated. Aluminum blister foil packaging is made with COC, which is a material that is used in the manufacturing process. This knowledge is only a couple of years old at most. Because COC films have superior moisture and gas barrier properties, tablets can be protected from environmental influences by using them.
It has excellent barrier properties against moisture, oxygen, and light. It also has excellent barrier properties against light. Additionally, it has excellent properties as a barrier against light. It is able to prevent these three components from functioning properly. In the manufacturing of backing cards for blister packaging, paperboard is a material that is frequently used and can be found in some instances. In order to complete this process, you may need to use a variety of paperboard-based materials.
A number of factors, including the specific requirements of the tablets, conformity with the requirements of the regulatory authorities, the stability of the product, and the level of protection that is desired, play a role in determining the material that will be used.